Automated Tissue Cell Processing Lab
KISO Processor

Automated Cell Lab
KEAI Bioresearch Inc. is a biotechnology company pushing the frontiers of medical research and clinical sciences. Our vision started 5 years ago with the belief that innovative thinking, bold and courageous leadership, personal dedication and the utilization of cutting edge technology would result in the creation of a platform technology that could change the future of medical research and in vitro diagnostics.

Introducing KISO system
The KISO system allows physicians, diagnostic laboratories and researchers to take human tissue and automatically isolate a consistent, high quality, potent unit of rich regenerative cells, cell isolates or target microorganisms for in vitro diagnostics. The entire process is completely standardized and automated in a closed system.

Compact & Standardized
Our easy to use proprietary patented technology is designed for applications where safe, reproducible, quality controlled products are desired. This results in superior recovery of minimally manipulated cells of pristine quality and purity within an hour of tissue harvesting.
Your In House Automated System
Step 1 - Input Sample ID (20 sec)
Step 2 - Load Disposable Set (1 min)
Step 3 - Load Lipoaspirate (20 sec)
Step 4 - Push "START" (1 sec)
Step 5 - Retrieve (47 min)
Step 6 - Discard Disposable Set (20 sec)
What We Do
Demand Only the Best Cells and Best Prepared Tissue Samples for Invitro Diagnostics

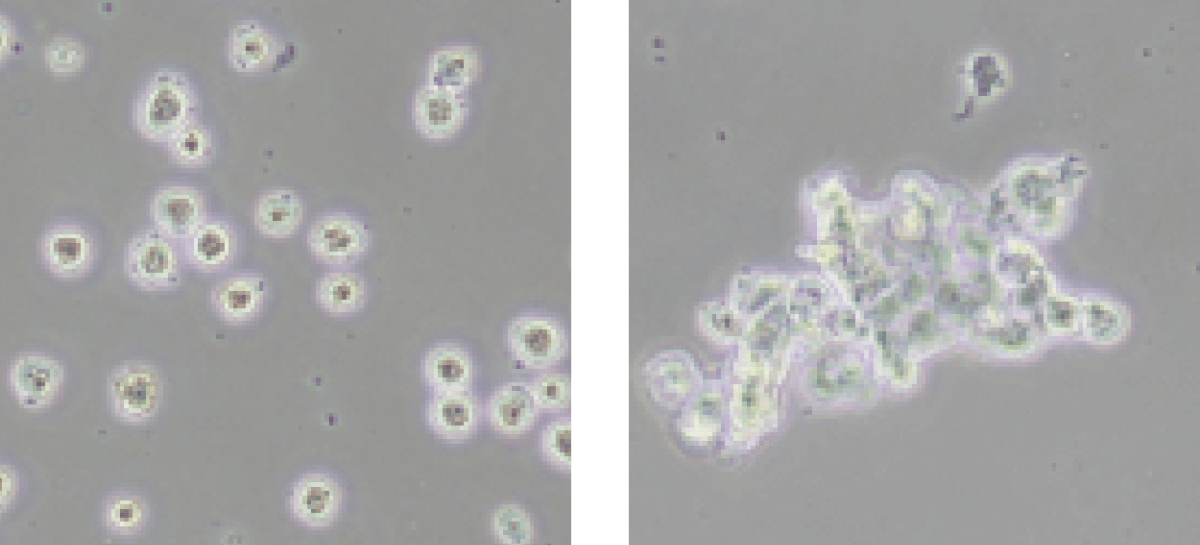
Highest Quality Cells, Clump Free! (left)
Our system provides highly potent & viable stem cells for the best possible outcome. Say good bye to cell clumps and clogged needles!
Our multi-step processing (enzyme or enzyme free) provides the best cells possible. This means cleaner research projects and a smoother mixture of regenerative cells for a wide range of applications.
Competitor's Output (right)
Clumps of dead cells are not desired and will jam needles on injections or cause serious process complications

Real Cell Counts vs Cell Counts
Not all count data provided is comparable
A cell count should provide you with viable nucleated cells and not a total count that includes non-viable cells. Additionally, competitors samples are known to be highly contaminated with a high level of white blood cells - artificially increasing the cell counts. Additionally, the majority of modern culture techniques still take a colony-forming unit-fibroblasts (CFU-F) approach for determining the count of Mesenchymal stem cells, or MSCs - that can differentiate into a variety of cell types. This MSC count data is often unavailable from competitors and providers
of stem cell procedures as it has been shown in academic literature to be very low vs our high MSCs counts.
Viable Nucleated Cells
Ask for the counts. I'll show you mine - you show me yours! Don't settle for less.
801,000 ± 167,000 viable nucleated cells from each gram of human lipoaspirate tissue (mean ± SD, n=7, median: 786,000 viable nucleated cells/g), with a range of 570,000 – 1,060,000 viable nucleated cells/g. The viability of cells recovered: 84.8% ± 3.8%. The high viability is an indicator and the gentle handling and isolation of the cells.
Higher viability means gentler handling of the cells, greater potency -> significant medical outcomes. New trials with our platform will lead to new outcomes never seen before.
7.2% ± 2.4% or 57,700 MSCs/g of the viable nucleated cells in the output our our system are cells MSCs as characterized by colony-forming unit-fibroblasts (“CFU-F”), compared to the reported 0.2% – 1.7% achieved by competitor systems. With the significantly higher viable cell recovery and stem cell colony counts, our system can produce 14x to 640x more MSCs, from each gram of human lipoaspirate vs the leading competitors.
Extreme Standardization
Regardless of where research or cell therapy is conducted, medicine requires standardization and performance reproducibility.
Our resulting measure of machine-to-machine variability was 2.4%, suggesting that the systems can perform stem cell extraction with exceptional consistency and reproducibility - eliminating any doubt in whether you are getting stem cells or not in your procedure.
Ease of Use and Speed Matters
Fast and furious "gentle" processing in 50 minutes
Extremely easy to operate with basic orientation and training. Once the isolation process starts, walk away and wait for you cells to be freshly and gently isolated.
Unrealized until now.
Indispensable from now on.
A better, faster stem cell processor.

22x
KISO produces over 22X more viable cells per gram of adipose tissue compared to Lipokit.+
KISO extracts as many cells from 60ml of adipose tissue as Lipokit does from 1 liter – automatically, safely, and at a lower total cost.*
+Saving costs include labour, capital costs, sterilization, disposables, admin efficiencies and reduced quantity of reagents (research or GMP grade).
*KISO competitor data from J. Aronowitz, Plast Reconstr Surg. 132(6):932e-9e, 2013
KISO Processor
The first desktop stem cell lab in the world.
Specification
Footprint: 630mm x 280mm
Height: 460 mm
Weight: 20kg Input
Voltage: 100 – 245 VAC 50/60Hz
Power Consumption: 100W
Display: 7” color touch screen panel
Optimal Ambient Temp: 20ºC – 40ºC
Process Time: 50 minutes
Contact Us
Keai Bioresearch Inc.
2270-8788 McKim Way
Richmond, BC V6X 4E2 Canada
Designed and Manufactured in Canada.
Unless stated otherwise, the products and services on this website are for Laboratory and Clinical Diagnostics Use Only.
Copyright© 2018 KEAI Bioreserach Inc. All rights reserved.